Your Cart is empty
Fungi in Respiratory Samples of Horses with Inflammatory Airway Disease
Julie Dauvillier, Fe ter Woort, Emmanuelle van Erck‐Westergren
First published: 21 December 2018
Abstract
Background
Fungi contribute to the inflammatory response of lungs in horses with recurrent airway obstruction and in some forms of asthma in humans. The role of fungi in inflammatory airway disease (IAD) has not been assessed.
Objectives
Evaluate the prevalence of fungi in the respiratory samples of horses diagnosed with IAD, describe clinical signs associated with the presence of fungi in respiratory samples, and assess the risk factors associated with IAD and with the presence of fungi in the airways.
Animals
Seven‐hundred thirty‐one active horses referred to a specialized ambulatory practice for signs of respiratory disease or poor performance.
Methods
A prospective observational study was performed, collecting clinical data, environmental conditions, and results of a tracheal wash (TW; cytology, fungal culture, and bacterial culture), and bronchoalveolar lavage (cytology).
Results
A positive fungal culture was obtained in 55% (402/731) of horses. Horses with fungal elements observed on the TW cytology had 2 times greater chance of having IAD than horses without fungi (odds ratio [OR] = 2.1; 95% CI 1.08‐3.33; P = .0003). Risks of being diagnosed with IAD and likelihood of fungi in TW were higher when horses were bedded on straw (OR = 2.0; 95% CI 1.2‐3.2 and OR = 1.9; 95% CI 1.3‐2.6, respectively) or fed dry hay (OR = 2.7; 95% CI 1.7‐4.4 and OR = 2.6; 95% CI 1.6‐3.4, respectively).
Conclusions and Clinical Importance
Horses inhaling aerosolized fungal particles are at a significantly higher risk of developing IAD. The type of bedding and forage represent significant risk factors for IAD and fungal contamination of equine airways.
Abbreviations
- ABPA - allergic bronchopulmonary aspergillosis
- ACVIM - American College of Veterinary Internal Medicine
- BAL - bronchoalveolar lavage
- BALF - bronchoalveolar lavage fluid
- CI - confidence interval
- CS - consensus statement
- IAD - inflammatory airway disease
- OR - odds ratio
- RAO - recurrent airway obstruction
- TW - tracheal wash
1 INTRODUCTION
Inflammatory airway disease (IAD) is a common cause of respiratory disease and poor performance in horses. Recently, it has been suggested that IAD should be included in the term “Equine Asthma,” regrouping both IAD and the more severe recurrent airway obstruction (RAO) as both are believed to represent a continuous spectrum of IADs in horses. There is a close parallelism between these forms of equine disease and human asthma.
Environmental factors play a pivotal role in the development of respiratory diseases. Horses that are housed indoors are exposed to high levels of organic dust, which include bacteria and fungi. Materials such as hay and straw naturally contain a substantial indigenous microflora that can further proliferate when stored or when used as forage or bedding.
The constitutive elements of fungi such as spores, conidiae, and hyphae are particles small enough to be inhaled up to the alveolar level and can trigger an immune response.
Fungi that penetrate the airways can be infective, toxic, allergenic, or all 3 combined. Some mesophilic fungi can only grow at environmental temperatures of 18°C to 22°C and rarely cause infection but are recognized allergens (Cladosporium and Alternaria). Other more thermotolerant fungi can grow at both environmental and body temperatures and thus act as both aeroallergens and opportunistic pathogens (Candida, Penicillium, and Aspergillus). When infective, they can further damage their host by producing toxins. Aspergillus species are the most prominent cause of fungal lung infections in humans.
Statements from different medical associations have unequivocally established that fungi act both in sensitizing and exacerbating allergic asthma in humans. Fungal pathogenicity is of particular importance in patients with severe asthma caused by fungal sensitization and patients with allergic bronchopulmonary aspergillosis (ABPA). In one third of these patients, antifungal treatment enabled to downgrade the severity of their asthma. However, Aspergillus and other fungi can also be sensitizers in other less severe forms of asthma, and many recent studies further describe the mold‐related respiratory effects in asthma. Farmer's lung is a well‐known disease of people working in an agricultural environment. It is linked to the inhalation of dust including mold spores. It has been shown that people working in horse stables were also at an increased risk of developing respiratory diseases, including asthma.
Molds have long been recognized to play a role in the pathogenesis of RAO in horses. Certain species of thermophilic actinomycetes such as Aspergillus fumigatus have been incriminated in the pathogenesis of both RAO in horses and Farmer's lung in humans. The inhalation of a combination of molds and endotoxin exacerbates RAO signs, including loss of respiratory function in experimental models. The involvement of fungi in the pathogenesis of IAD remains unclear. As in RAO, exposure to inhaled dust and allergens could play a critical role in the development of the disease. The association of various dust components found in stable air with IAD has been investigated and includes organic dust, endotoxin, and ammonia, but no link has been made between IAD and exposure to fungal particles.
The purpose of our prospective observational clinical study was:
to evaluate the prevalence of fungal isolates in the respiratory samples of horses diagnosed with IAD, as well as in healthy horses,to describe clinical signs associated with the presence of fungi in respiratory samples of horses diagnosed with IAD, andto assess risk factors associated with IAD and the presence of fungi in the airways.
2 MATERIALS AND METHODS
2.1 Horses
The study was performed in a population of active sport, race, and leisure horses based in Belgium, France, and the Netherlands. A total of 731 cases referred to a specialized ambulatory internal medicine practice were assessed between June 2013 and July 2016. The horses were referred for respiratory disease, for decreased performance, or in some cases, for a routine seasonal examination without any clinical complaint. For each case, a clinical examination, an airway endoscopy, a tracheal wash (TW), and a bronchoalveolar lavage (BAL) were performed. Data regarding the type of bedding (straw, wood shavings, and other) and hay forage (dry hay, moistened hay, damped hay, steamed hay, or haylage) were systematically collected.
2.2 Respiratory sampling
Horses were sedated using either 0.03‐0.04 mg/kg IV romifidine chlorhydrate (Sedivet, Boehringer Ingelheim, Ingelheim, Germany) or 0.01 mg/kg IV detomidine chlorhydrate (Domosedan, Zoetis, Louvain‐la‐Neuve, Belgium) based on withdrawal time considerations. In horses with more marked clinical signs or in horses that were difficult to handle, 0.01‐0.02 mg/kg IV butorphanol (Dolorex, Intervet) was added to the alpha 2‐agonist drug.
2.2.1 Tracheal wash
A 120‐cm video endoscope (PE Optomed, Les Ullis, France) was passed through one of the nostrils into the mid‐trachea. The quantity of mucus in the trachea (grade 0‐5) was graded according to a previously published scale. A sterile catheter was passed through the canal of the endoscope, and 20 mL of sterile saline solution (0.9% NaCl) was infused and retrieved. The aspirated fluid was divided among a sterile tube for bacteriological analysis, a tube containing a Sabouraud medium for mycological analysis, and a tube containing ethylenediaminetetraacetic acide (EDTA) for cytological analysis. Equipment was sterilized between each case.
2.2.2 Broncholaveolar lavage
A sterile 3‐m BAL catheter (large animal BAL catheter; Mila International, Inc, Florence, Kentucky) was inserted in one of the nostrils and passed into the trachea until wedged in a bronchi. Then, 180 mL of sterile saline solution (0.9% NaCl) was sequentially infused and retrieved using sterile 60 mL syringes. Four milliliter of the fluid obtained in the last syringe was placed into an EDTA tube for cytological analysis.
2.2.3 Sample analysis
A cytological examination of both TW and BAL was made. The TW was submitted for further bacteriological and mycological cultures within 24 hours. Cytological analysis of TW and BAL was performed using a modified sedimentation technique. Briefly, the EDTA tubes were maintained in a vertical position for 2 hours to allow sedimentation. The supernatant was discarded and 0.6 μL of the sediment was pipetted onto a glass slide and dried. The slide was stained using a modified Wright‐Giemsa stain. The differential leukocyte count was performed on a minimum of 200 cells. The presence of fungal particles (spores, conidia, conidiophores, hyphae, mycelia, etc), spore germination, or branching hyphae were documented (Photos 1-3).
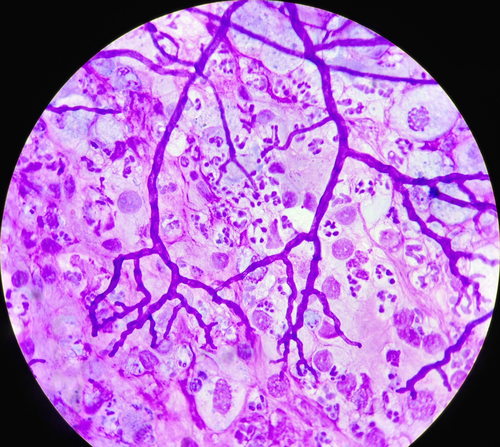
Photo 1
Branching hyphae on a bronchoalveolar lavage cytology (×400, modified Wright‐Giemsa staining)
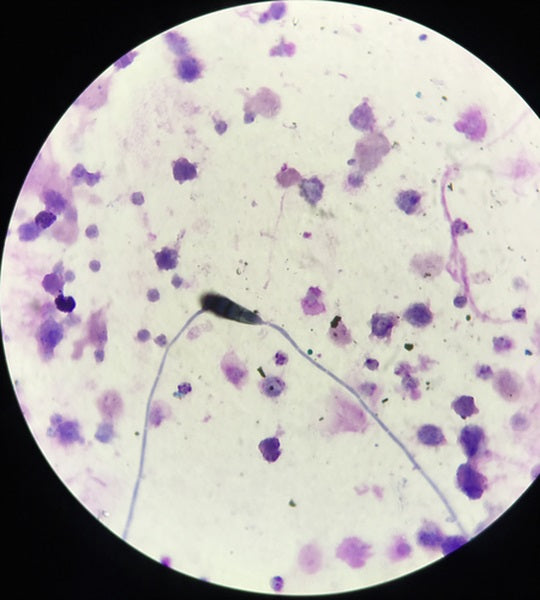
Photo 2
Spore germination on a tracheal wash cytology (×400, modified Wright‐Giemsa staining)
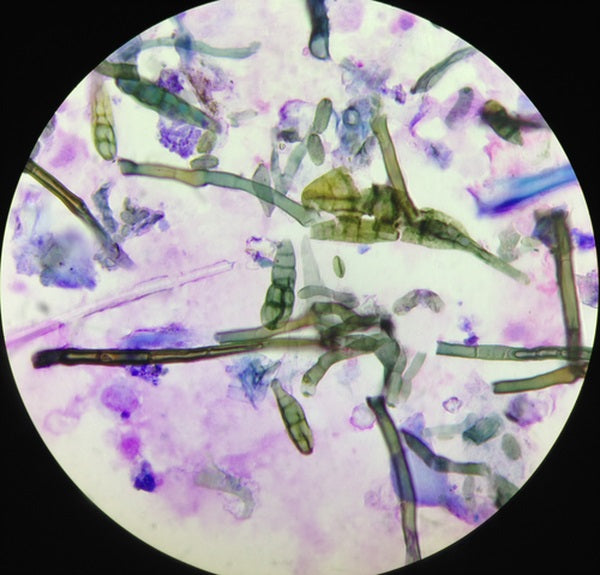
Photo 3
Fungal elements on a tracheal wash cytology (×400, modified Wright‐Giemsa staining)
2.3 Case definition
Based on the 2016 American College of Veterinary Internal Medicine (ACVIM) consensus statement (CS), a diagnosis of IAD was made based on the presence of clinical signs (poor performance, chronic occasional coughing, or both), evidence of airway inflammation based on BAL fluid (BALF) cytology, and the exclusion of severe equine asthma (RAO) based on history and clinical signs. The presence or absence of tracheal mucus detected by endoscopy was not used to make the diagnosis of IAD in our study.
However, regarding the cytology, the authors decided to use the more selective 2007 CS cutoff values for BALF cytology as they apply better to the high‐level sport and race horses included in our study. Indeed, performing horses of this level present BAL cytology lower than 2007 CS cutoff values (van Erck, unpublished data). Bronchoalveolar lavage fluid cytology was thus considered inflammatory if it contained more than 1% eosinophils, more than 2% mastocytes, more than 5% neutrophils, or any combination of the above.
The presence of fungal elements (spores, hyphae particles, or both) and signs of active growth (germinating spores, branching hyphae, or both) were recorded on both TW and BAL cytological samples.
The control group consisted of horses with or without clinical signs with a normal BAL cytology. Horses under corticotherapy or antimicrobial medication at the time of the examination were excluded from the study.
2.4 Statistical analysis
Descriptive summary statistics were reported for the overall set of IAD cases and controls. The sensitivity and specificity of clinical signs for the presence of fungal elements in the TW were calculated. Multivariate logistic regression models were developed to explore the relative contributions on IAD of the presence of fungal particles in the TW, BAL, or both after adjusting for potential risk factors (ie, age, sex, bedding type, hay type, and discipline). Interaction between risk factors and the presence of fungal particles were also considered in the models. Variables were selected with a stepwise selection procedure in which significance levels of the chi‐square tests for entering and removing a variable of the model were set at 5%. We also verified that no parameter estimate changed by more than 20% during the iterative process. Odds ratio (OR) and 95% Wald confidence interval (CI) limits were estimated for all variables included in the final model. A Cohen's kappa test was used to compare the agreement between fungal detection on cytology and mycology culture. Differences in inflammatory neutrophil cell counts between different beddings or forages were compared using a nonparametric Mann‐Whitney test. For all statistical tests, significance was set for P < .05.
All computations were done on SAS V.9.1 (Statistical Analysis System; SAS Institute GmbH, Heidelberg, Germany) (proc FREQ and proc LOGISTIC).
3 RESULTS
In the population studied, horses were aged 8.5 ± 4.3 years (mean ± SD), IAD‐positive (IAD+) horses were aged 8.9 ± 4.8 years, and control (IAD) horses were aged 8.3 ± 4.0 years. There was no effect of age or sex on the diagnosis of IAD. A diagnosis of IAD was established in 88% of cases (646/731 horses). The population of 731 horses was composed of 369 (50.5%) showjumpers, 87 (11.9%) dressage horses, 70 (9.6%) Thoroughbreds, 55 (7.5%) Standardbreds, 50 (6.8%) endurance horses, 35 (4.8%) eventers, and 65 (8.9%) leisure horses.
3.1 Fungal and bacterial data
A positive TW mycology culture was obtained in 55% of all horses (401/731), in 55% (356/646) of IAD+ cases, and in 52% (45/85) of IAD− horses. The most commonly isolated fungi were Penicillium (53%), Aspergillus (34%), Rhizomucor (5%), and Candida (5%).
Overall, fungal elements were found upon cytological examination of the TW in 79% (578/731) of all horses, in 81% (523/646) of IAD+ horses, and in 65% (55/85) of IAD− horses. This difference was statistically significant (P = .0006). Fungal elements were identified in both TW and BAL in 245 of 731 (33.5%) horses, only in the TW in 332 of 731 (45.4%) horses, and only in the BAL in 28 of 731 (3.8%) horses. For the statistical analysis, the presence of fungal elements in the TW was used, as this reflects more accurately the overall exposure of the equine airways to these particles.
Comparing mycology culture to fungal detection on cytology, the agreement between both techniques was poor (Cohen's kappa coefficient κ = 0.063; 55.9% agreement). Horses with fungal elements in their TW on cytology had 80% increased odds of having a positive TW mycology (OR = 1.8; 95% CI 1.2‐2.5; P = .002). However, no correlation was found between a positive mycology and the detection of fungal growth on cytological examination. A positive TW bacterial culture increased by 2.1‐fold the chances of finding a positive TW mycology culture (OR = 2.1; 95% CI 1.5‐2.8; P < .0001).
3.2 Relationship with IAD
Horses with fungal elements on the TW cytology, whether they proliferated or not and whichever fungal species considered, had 2.1‐fold more chances of having IAD than horses with no fungi (OR = 2.1; 95% CI 1.08‐3.33; P = .0003). The presence of fungi on the TW cytology was not statistically associated with a particular cytological phenotype. Positive bacteriology or mycology of the TW as well as fungal growth were not associated with a positive IAD diagnosis.
3.3 Associated clinical signs
Distinctive respiratory clinical signs such as cough, breathlessness, or nasal discharge had low sensitivity but moderate to high specificity for the presence of fungi (Table 1).
Table 1. Specificity and sensitivity of respiratory clinical signs for the presence of fungal elements in TW of horses
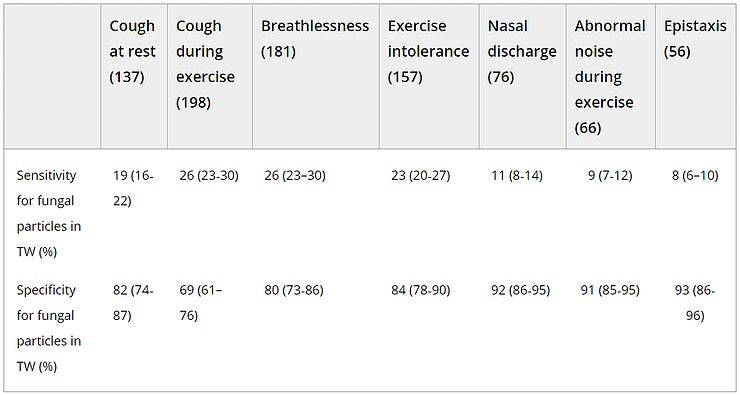
- Abbreviation: TW, tracheal wash.
- A complaint of exercise intolerance was more frequent in horses with TW fungal particles (23%) versus horses without (16%; P = .05). The frequency of cough at rest, cough at work, exercise intolerance, and nasal discharge was higher in horses with a positive mycology culture (21%, 30%, 24%, and 12%, respectively) versus horses without (15% [P = .04], 23% [P= .03], 18% [P = .04], and 8% [P = .04], respectively).
- The ACVIM revised CS on IAD established that a diagnosis of IAD can be made on the basis of the endoscopic detection of excess tracheal mucus, with a score >1/5 in racehorses and score >2/5 in sport and leisure horses. Analysis of the results on 667 horses on which the tracheal mucus score was available indicated that the mucus score test for the diagnosis of IAD showed poor sensitivity in both sport and race horses (48%, 95% CI 43‐52; and 49%, 95% CI 39‐60, respectively). The specificity of this test was high in sport horses (90%; 95% CI 79‐96) but more moderate in racehorses (73%; 95% CI 45‐92).
3.4 Environment
The degree of lower airway inflammation (% neutrophils in BAL) was significantly higher when horses were housed indoors and bedded on straw versus shavings (25.5 ± 17.1 versus 19.9 ± 17.5; P < .001) or when fed dry hay versus steamed hay (23.2 ± 17.5 versus 18.8 ± 18.9; P < .001).
Horses bedded on wood shavings had 40% reduced odds of having fungal elements in their TW (OR = 0.6; 95% CI 0.4‐0.9) and 30% reduced odds of being diagnosed with IAD in comparison to horses on other bedding types (OR = 0.7; 95% CI 0.5‐1.2). Conversely, horses housed on straw had 90% increased odds of having fungal elements in their TW (OR = 1.9; 95% CI 1.3‐2.6) and twice more chances of being diagnosed with IAD (OR = 2.0; 95% CI 1.2‐3.2) than horses housed on other bedding types.
Horses fed with dry hay had 2.6 times more chances of having fungal elements in their TW (OR = 2.6; 95% CI 1.6‐3.4) and 2.7 times more chances of being diagnosed with IAD (OR = 2.7; 95% CI 1.7‐4.4) whereas horses fed with steamed hay had 65% reduced odds of being diagnosed with IAD (OR = 0.35; 95% CI 0.2‐0.8). The alternatives to dry hay other than steaming, such as wetting or soaking the hay (OR = 2.4; 95% CI 1.2‐4.8), feeding haylage (OR = 1.6; 95% CI 0.7‐3.3), or commercial “dust‐free” hay (OR = 1.6; 95% CI 0.6‐4.0), all increased the risks of having a positive IAD diagnosis (Figures 1 and 2). Soaking the hay did not influence the finding of fungal elements in the TW; however, steaming hay (Haygain HG; Haygain Ltd, Lambourne, UK) decreased by 2‐fold the risks of having fungal elements in the TW (OR = 0.5; 95% CI 0.3‐0.8).
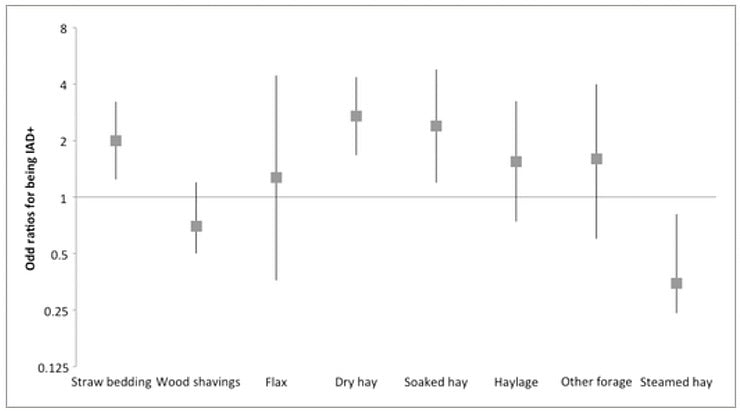
Figure 1
Odd ratios (bars represent 95% confidence interval) of being diagnosed with inflammatory airway disease depending on the bedding and forage type
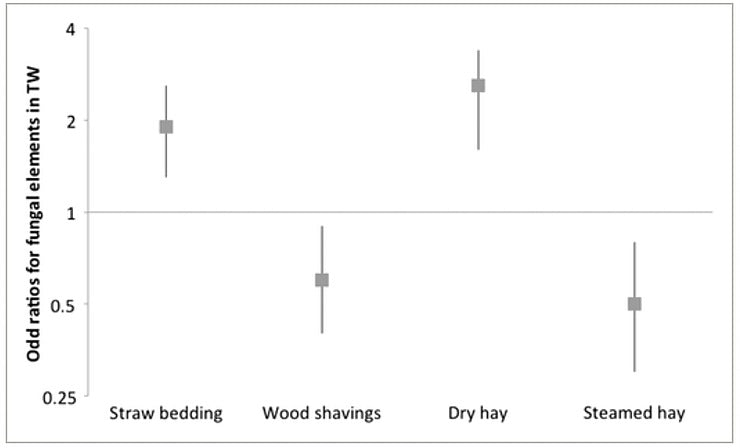
Figure 2
Odds ratios (bars represent 95% confidence interval) of finding fungal elements in the tracheal wash depending on bedding and forage type
4 DISCUSSION
Our study demonstrates that fungal elements are commonly present in equine airways and that horses inhaling aerosolized fungal particles are at a significantly higher risk of having IAD. These findings support the role that aerosolized fungal elements play in the pathophysiology of IAD, as it is the case in RAO. Fungi can be allergenic, infective, toxic, or any combination of the above. The role of fungi in equine IAD needs to be further investigated. Although fungi might not necessarily be the primary cause of IAD, it is possible that deficits in immunity might modify the equine ability to react to infection. In humans, in addition to asthma or invasive fungal pneumonia, other more moderate fungal infections have been described, such as chronic pulmonary aspergillosis, aspergillus bronchitis, ABPA, and severe asthma with fungal sensitization. The results of our study suggest that this category of intermediate pathological fungal conditions also exists in the airways of horses as well. The identification of proliferating fungi in the airways is a sign of fungal infection and warrants further investigation.
General factors promoting fungal development in people include decreased immune function, treatment with corticosteroids and antibiotics. Decreased immune function has also been demonstrated in horses undergoing intense exercise and stress associated with transportation and competition. A link between fungal growth and an immunodepressive state could not be demonstrated in our study; however, it is likely that the immune system of some of the horses included in our study would have been challenged by intensive training, regular transport, and competition.
The high frequency of fungal particles in the airways of horses raises the question of the safety of use of corticosteroids as a unique treatment of airway inflammation and the risks of promoting fungal growth by depressing the immune response in the airways. In human allergic diseases involving fungal component such as severe acute respiratory syndrome and ABPA, antifungal treatment is added to the treatment plan.
Furthermore, in these authors' experience, indiscriminate use of antimicrobials can occur in the sport horse population, potentially increasing the risk of respiratory fungal growth. In our study, no relationship could be established between a positive bacteriology of the TW and a diagnosis of IAD. This supports the noninfectious etiology of IAD and questions the empirical use of antibiotic treatments in the management of IAD or in cases of cough or nasal discharge.
Horses with a positive bacterial culture of the TW had a greater risk of a positive mycology culture. This raises the question about a possible link between bacterial and fungal infections. In people, airway inflammation leading to decreased mucus clearance can lead to environmental bacteria getting trapped within the mucus and not being eliminated normally which promotes infection. The same could be true for fungi. In people, fungi like Aspergillus are suspected to decrease the local immune response and mucus clearance via the secretion of mycotoxins like gliotoxin and verruculogen. These mycotoxins have shown to damage human epithelial cells and modify the electrophysiological properties of human or porcine epithelial cells in vitro.
Fungal culture and cytological detection of fungi showed poor agreement. In a number of cases, fungal culture failed to detect fungal presence in the airways. Interestingly, fungal culture also failed to identify several cases that showed fungal growth on cytology. Thus, mycology was not a sensitive method to detect horses with a more severe fungal condition. Although no relationship could be established between fungal growth and a diagnosis of IAD, horses with developing hyphae in their airways should be cautiously evaluated as it has been shown that most fungal allergens are released after spores germinate. In people, routine fungal culture methods underestimate the presence of fungi in respiratory samples. When comparing the cytological detection of fungal elements with fungal culture in respiratory samples of patients who died of pulmonary aspergillosis, cytology was found to have superior sensitivity and specificity, using postmortem as the gold standard reference. In the context of a diagnosis of IAD, the authors recommend that both cytology and mycology be performed on the TW. Identification of fungal elements in the TW was far more frequent than in the BAL. Although not useful for the diagnosis of IAD in itself, a cytological examination of the TW might be a good way to evaluate exposure of the horse to fungal particles in this environment.
The most commonly isolated fungi in this population were Penicillium (53%), Aspergillus(34%), Rhizomucor (5%), and Candida (5%). Penicillium, Aspergillus, and Muroraceae species are airborne and ubiquitous and classically found in stable air. Aspergillus is the most common isolated fungi in hay.
Spores and fragments from Aspergillus and Penicillium species are small enough to reach the lower airways (2‐10 μm); because they are thermotolerant, they are also able to germinate and colonize the region. These species are the ones most commonly associated with fungal polysensitization in humans.
Although many studies have demonstrated the link between environmental factors and RAO, no such studies are available for IAD. Our study showed that clinical signs such as cough, breathlessness, and nasal discharge were poorly sensitive for the diagnosis of IAD and for the presence of fungal particles in the airways. However, cough and nasal discharge were more prevalent in horses with IAD or with a positive TW mycology.
Exercise intolerance was also significantly more prevalent in horses with a positive TW mycology. None of the horses showed the severe clinical signs typically described in the cases of fungal pneumonia (depression, deep cough, fever, anorexia, and weight loss). Thus, it is not possible to rely solely on clinical signs to diagnose IAD and the presence of fungal particles in the airways. Respiratory sample analysis is further indicated, and a cytologic examination appears more sensitive than a mycology culture to detect the presence of fungi.
The 2016 ACVIM revised CS stated that tracheal mucus can be part of the diagnosis of IAD, with excess tracheal mucus (score > 1 in racehorses and score > 2 in sport horses) observed upon endoscopy being a criteria just as valuable as BALF inflammation or abnormal lung function. In our study, this method would have led to numerous false‐positive and false‐negative cases suggesting that mucus endoscopic scoring is unreliable for diagnosing IAD.
In our study, straw bedding and dry hay feeding represented significant risk factors for IAD and for the presence of fungal elements in equine airways. Their use cannot be recommended in performance horses. Fungal spores naturally contaminate hay and straw during harvest. The storage of hay and straw can also lead to an exponential increase in fungal proliferation within the batches. The degree of contamination and proliferation is directly related to harvesting practices, initial levels of soil contamination, as well as storage conditions. On the contrary, wood shavings decreased the risk of IAD and the detection of fungal particles in the airways. This bedding type seems to be an appropriate solution to maintain equine respiratory health. The use of high‐temperature hay steaming also had a significantly protective effect against the development of IAD and the contamination of airways with fungal particles in our study. Hay steaming has been shown to significantly reduce bacterial as well as fungal contamination and could be an effective means to improve the hygiene of forage. Interestingly, soaking the hay, which is often recommended as a protective measure for horses with respiratory inflammation, did not significantly decrease the risk of being IAD nor the risk of having fungal elements in the airways. Similarly, the use of haylage did not reduce the risk of IAD in our study. The absence of a significantly preventative effect of haylage could be attributable to its use in a limited number of horses and the large variation in the quality of haylage products used. Similarly, hay commercialized as “dust‐free” hay was not a good alternative in our study as it did not reduce the risk of IAD nor the risk of finding fungal particles in the airways.
The prevalence of positive IAD cases was very high in the studied population but comparable to values previously reported in middle‐aged competitive horses. This could be partly attributed to the profile of the referral practice, which is specialized in sports and internal medicine and has a high caseload in poor performance and respiratory issues. The vast majority of horses (721/731) were housed indoors, which is an identified risk factor for IAD. The fact that all horses were in active training and a majority of them regularly involved in competitions contributed to increasing their exposure to identified risk factors for IAD (transport, comingling, and immune challenge linked to intense exercise).
As this is an observational study, no conclusion could be drawn regarding the underlying physiopathology associating the presence of fungi and respiratory disease.
In conclusion, IAD is a highly prevalent disease in active sport horses that is typically associated with poor performance rather than specific respiratory clinical signs. Fungi present in the equine environment can be inhaled in the airways; they represent a significant risk factor for IAD and contribute to exercise intolerance. The identification of these fungal elements should be made on the basis of TW cytology slides as many of these cases were negative on mycology culture. The quality of the environment and management practices are determinant in reducing exposure to contaminants and maximizing the equine respiratory health. The role of fungi in the pathogenesis of IAD and equine asthma should be further investigated.
ACKNOWLEDGMENT
The authors acknowledge the contribution of Dr Johann Detilleux for her help with the statistics. The results of this study were presented in part at the 2016 ACVIM Forum, Denver, CO, and at the 2017 WEAS in Copenhagen.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflicts of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
The Original Article can be found here: https://onlinelibrary.wiley.com/doi/full/10.1111/jvim.15397
For information on Steaming hay and the Haygain Steamer units, see this link: https://www.amacron.com.au/haygain-steamers


